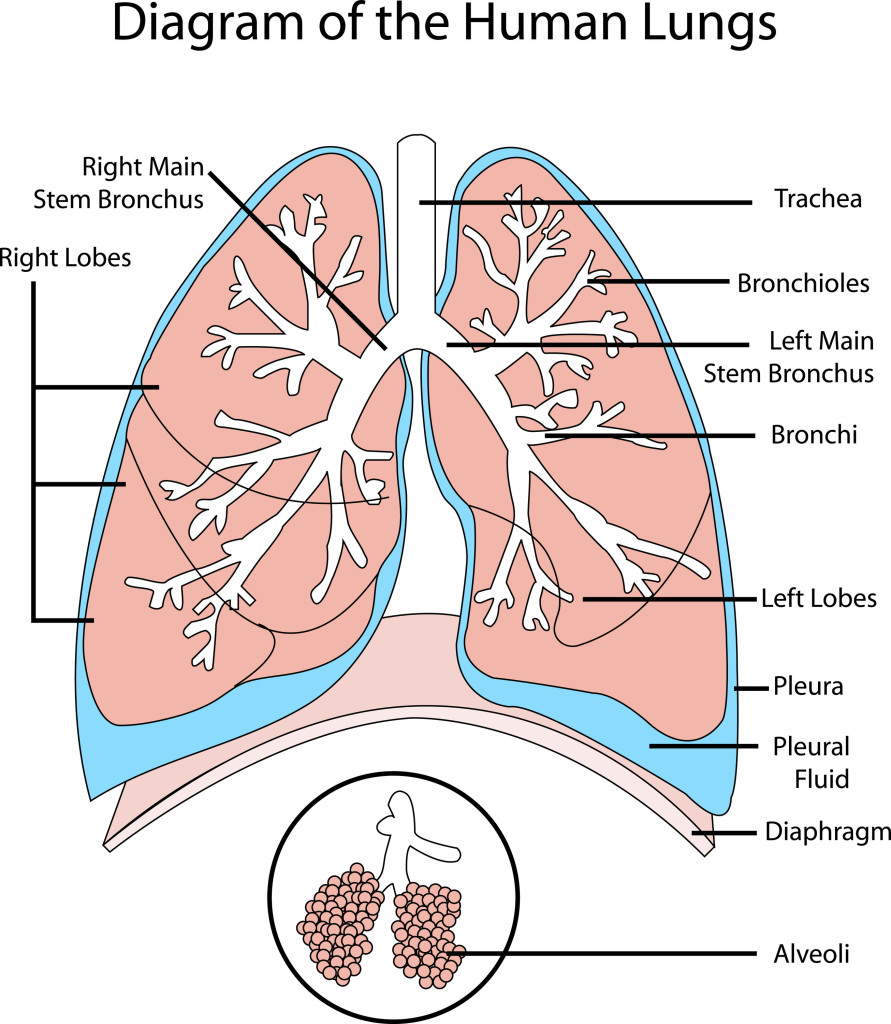
Normal Lung Anatomy
The lungs are surrounded by a double membrane called pleura. This membrane has two layers, a parietal pleura and a visceral pleura. The parietal pleura is attached to the chest wall and the visceral pleura covers the surface of the lungs. Between these layers of pleura is a potential space i.e. a space that doesn’t exist but it could exist if something goes wrong. There is a small amount of fluid in this potential space, this serous fluid acts like a lubricant.
The purpose of the pleura is to allow the lungs to move inside the body cavity without friction as friction would cause damage to the lungs and the tissue in the chest wall. Imagine that if you got an empty balloon and put a few drops of a lubricant such as olive oil inside it. You can rub the two sides of the balloon against each other very smoothly and easily.
Pressure and inspiration
The intrapleural pressure, that is, the pressure in between the two layers of pleural membrane is -4mmHg. What? But that’s not possible. How can you have negative pressure?
Technically, it’s not negative pressure, it’s just less than atmospheric pressure. (Atmospheric pressure is approximately 760mmHg, intrapleural pressure is 756mmHg.) This pressure difference keeps the lungs ‘stuck’ to the chest wall so they don’t collapse.
The lungs can expand because of compliance, the ability of the lungs to stretch. This is different to elasticity which is the tendency of your lungs to spring back to their original shape.
Healthy people’s lungs are always partly stretched, if they were fully collapsed, we would never be able to breathe. You might ask, “Why?” Because of surface tension.

Alveoli
As you may know, the lungs are filled with little sacs called alveoli; this is where gas exchange occurs. In order for this to happen, there is a thin layer of liquid on the surface of each alveoli to help the gases dissolve and move across the membrane. This poses a problem: surface tension.
You may have noticed surface tension before; a water droplet sitting on a leaf is dome shaped because of surface tension; all of the water molecules are attracted to each other. The problem with this is that too smooth wet surfaces that are stuck together are a lot harder to pull apart than if they were dry. If you’ve ever stuck two microscope slides together with water in the middle, they are very hard to separate.
This means that if an alveoli is completely collapsed, it is extremely hard to open again. To give it a bit of a hand, our alveolar cells make a substance called surfactant (this is a lipoprotein.). This substance lowers surface tension and makes it a lot easier for alveoli to open if they collapse. (A bit of trivia, babies that are born very prematurely can’t make surfactant and must be given artificial surfactants to help them breath.)
Even with surfactant, it still takes a lot of effort to open all the alveoli so it is advantageous not to have the lungs collapse fully.
So, how do we breathe?
You breathe by expanding your chest wall and contracting your diaphragm. Your diaphragm is a big thin muscle that sits under your lungs and heart. The diaphragm pulls your lungs downwards and your chest wall pulls outwards and upwards – this causes your lungs to stretch and pull air inside. When you relax, the elasticity of the lungs pushes air out.
I hope this has helped show how the lungs work to pull in air and the factors that aid them in keeping us alive.